Biosimilars: What They Are, How They Work, and Why They Matter
When you hear biosimilars, highly similar versions of complex biologic drugs that are made from living cells, not chemicals. Also known as biologic generics, they work just like the original drugs but cost far less. Unlike regular generics, which copy simple chemical pills, biosimilars match intricate proteins made by living organisms—like antibodies or hormones—used to treat cancer, rheumatoid arthritis, diabetes, and Crohn’s disease. They’re not exact copies, but they’re close enough that the FDA and other global agencies approve them as safe and effective replacements.
These drugs aren’t new, but their use is growing fast. Many people still think they’re risky or inferior, but studies show they perform just like the originals in real-world use. For example, biosimilars of adalimumab (Humira) and infliximab (Remicade) have helped millions cut monthly costs from over $2,000 to under $500 without losing effectiveness. That’s huge for patients on lifelong treatment. They’re also helping healthcare systems stretch budgets further, so more people get access to life-changing therapies.
What makes biosimilars different from regular generics? It’s the complexity. A regular pill has one known structure. A biologic is made by cells in a lab—tiny changes in temperature, pH, or process can alter the final product. That’s why manufacturers must prove biosimilars match the original in structure, function, safety, and how the body responds. No guessing. No shortcuts. Just hard science and rigorous testing. And because they’re not exact copies, they’re called biologic drugs, complex medicines derived from living organisms, often used for chronic autoimmune and cancer conditions—not generics.
Some doctors still hesitate to switch patients over, especially if someone’s stable on the brand-name drug. But that’s changing. Many insurance plans now push for biosimilars first, and clinical guidelines support the switch. If you’re on a biologic and your doctor suggests a biosimilar, ask: "Is this approved for my exact condition?" and "Has it been used safely in people like me?" The answer is usually yes.
There are also drug cost savings, the financial benefit patients and systems gain when biosimilars replace expensive brand-name biologics—often 20% to 40% lower. That’s not just a number. It means people can afford treatment longer. It means fewer skipped doses. It means fewer hospital visits from complications caused by skipping meds because they’re too expensive.
And here’s the thing: biosimilars don’t replace innovation. They make space for it. When cheaper options open up, money and resources can go into developing the next wave of treatments. Right now, over 30 biosimilars are approved in the U.S. alone, with dozens more in development for conditions like multiple sclerosis, psoriasis, and even rare blood disorders.
You’ll find posts here that dig into how biosimilars compare to originals, what to watch for when switching, why some patients still get brand-name drugs, and how to talk to your doctor about cost without sacrificing safety. Whether you’re a patient, caregiver, or just trying to understand why your prescription changed, this collection gives you real answers—not marketing.
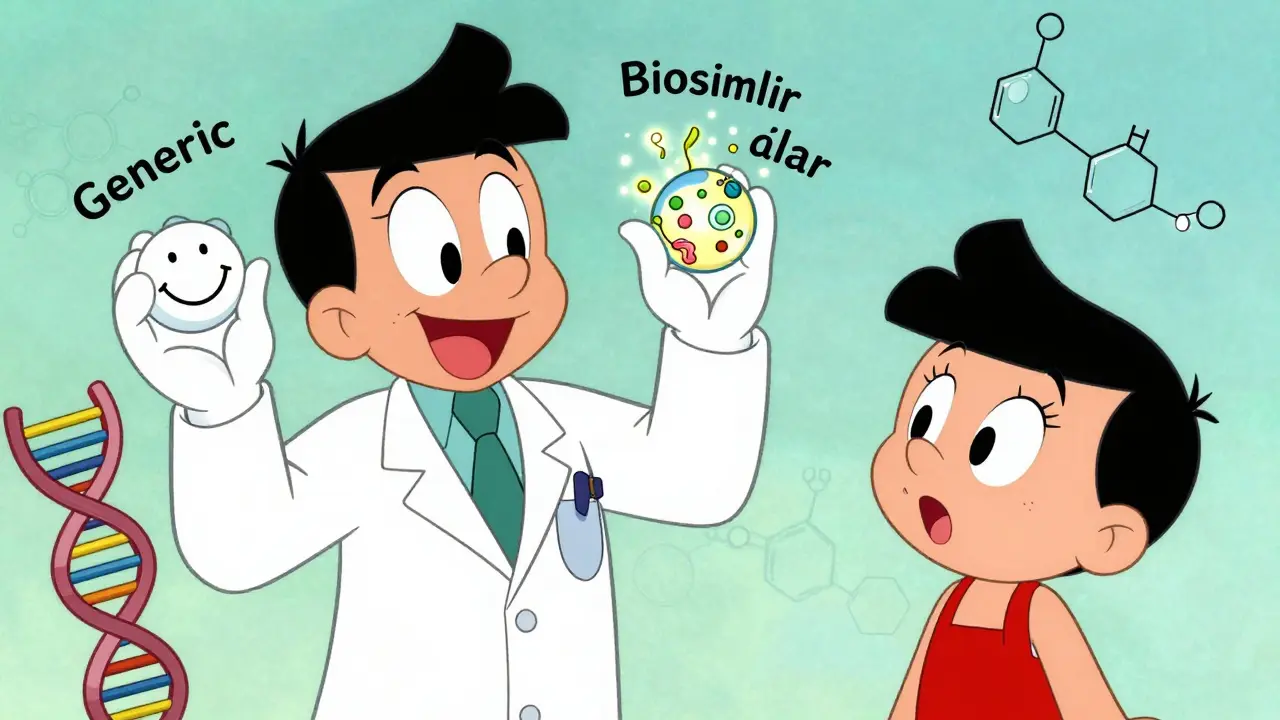
Biosimilar or Generic? How to Choose the Right Medication for Your Treatment
Learn the key differences between biosimilars and generic drugs to make informed treatment choices. Understand cost savings, safety, switching rules, and real-world effectiveness.
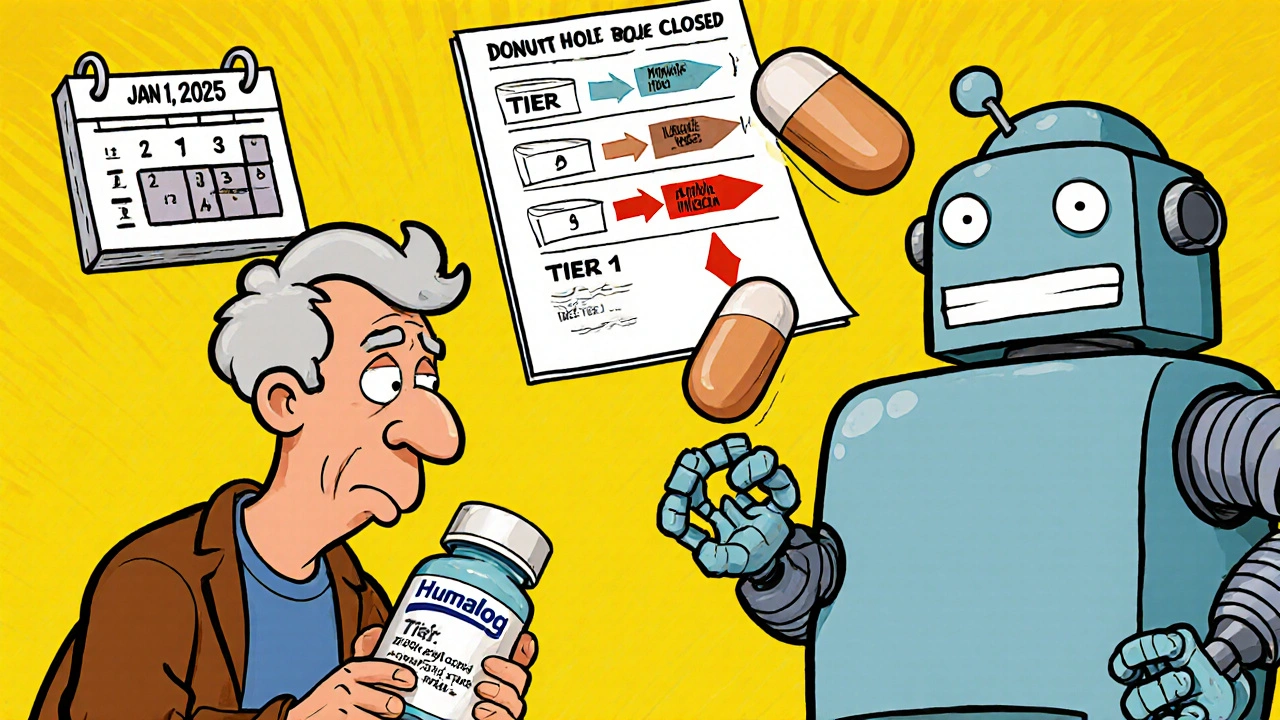
Insurance Changes and Generic Switching: How Formulary Updates Affect Your Prescription Costs in 2025
Understand how 2025 Medicare formulary updates are pushing patients toward generics and biosimilars, what it means for your prescription costs, and how to protect yourself from unexpected drug switches and price hikes.