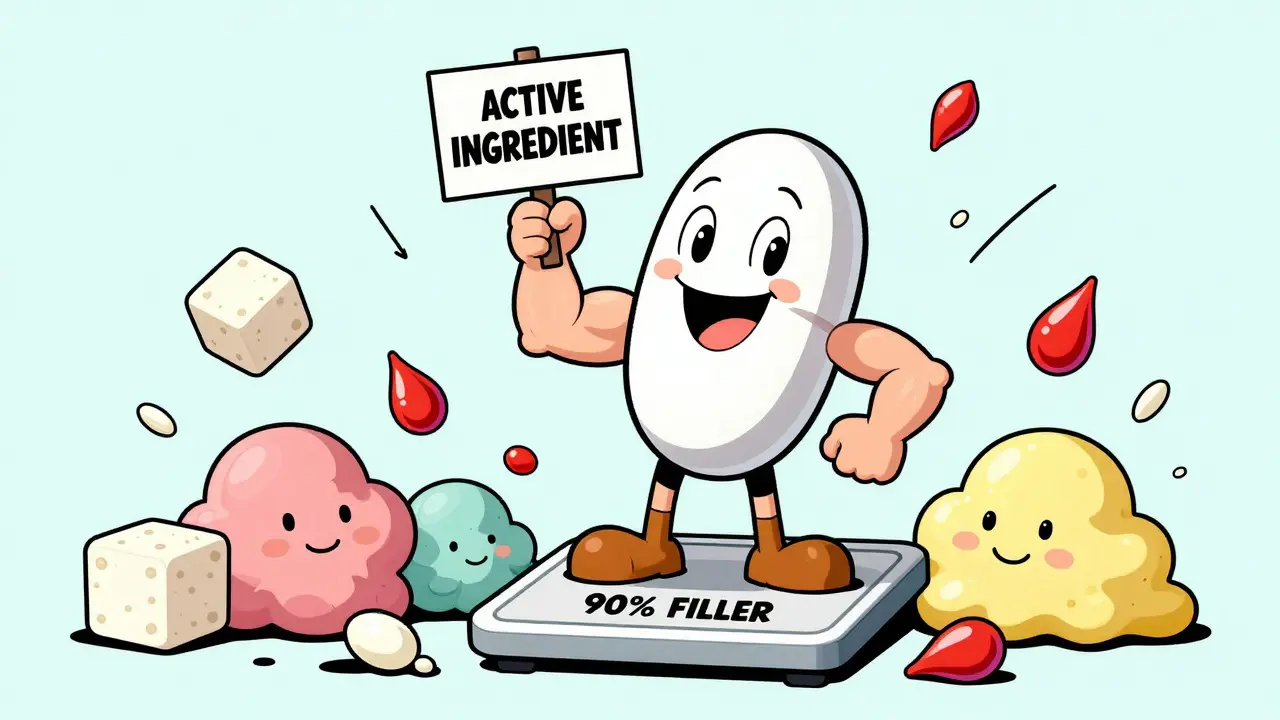
When you pick up a pill, you’re not just taking one thing. You’re swallowing a carefully engineered mix of two very different kinds of ingredients - and the difference between them can affect how well your medicine works, whether you have side effects, and even if it’s safe for you at all.
What’s actually doing the work?
The active ingredient is the part of the medicine that changes your body. It’s the reason you took the pill in the first place. In Tylenol, that’s acetaminophen. In Advil, it’s ibuprofen. In Lipitor, it’s atorvastatin. These chemicals interact with your cells, block enzymes, or bind to receptors to reduce pain, lower cholesterol, or fight infection. Without the active ingredient, the pill is just a fancy piece of sugar and starch. The U.S. Food and Drug Administration (FDA) defines active ingredients as components that have a direct effect on your body’s structure or function. That means they’re not just sitting there - they’re working. And because they’re so powerful, they go through years of testing before they’re approved. Over 90% of new active ingredients fail during clinical trials because they’re either unsafe or don’t work as needed.What are the other stuff in the pill?
The rest? That’s the inactive ingredients - also called excipients. They don’t treat your condition. But they’re still essential. Think of them as the support crew. They make sure the medicine can be made, stored, swallowed, and absorbed properly. Here’s what they actually do:- Fillers like lactose or microcrystalline cellulose give the pill size. If your active ingredient is only 5 milligrams, you need something to make the pill big enough to hold and swallow.
- Binders like gelatin or acacia hold everything together so the tablet doesn’t crumble.
- Lubricants like magnesium stearate keep the medicine from sticking to the machines during manufacturing.
- Coatings like hydroxypropyl methylcellulose help the pill go down smoothly and sometimes control when it releases in your gut.
- Preservatives like parabens stop bacteria or mold from growing in liquid medicines or multi-dose containers.
- Flavors and colors make it easier for kids - or adults - to take their medicine without gagging.
But are they really ‘inactive’?
Here’s where things get surprising. Just because an ingredient is called ‘inactive’ doesn’t mean it’s harmless - or even completely inert. A 2021 study from the University of California, San Francisco, and Novartis tested 639 FDA-approved inactive ingredients against over 3,000 human proteins. They found that about 14% of these so-called ‘inactive’ substances had unexpected biological activity. Some bound to proteins linked to inflammation, hormone regulation, or even cancer pathways. Take D&C Red 7 calcium lake - a common red dye. It showed strong binding to a protein involved in immune response. Propyl gallate, a preservative used in some liquid meds, interacted with enzymes that affect metabolism. These weren’t strong enough to be drugs themselves - but they weren’t harmless either. The FDA responded by launching the Excipient Safety Initiative in 2022, investing $4.2 million to study whether these ingredients might cause subtle, long-term effects - especially in people taking multiple medications daily for years.
Why this matters for you
You might think, ‘I’m not allergic to anything, so this doesn’t affect me.’ But that’s not always true. Lactose - a common filler - is a problem for about 65% of the world’s population. If you’re lactose intolerant, even a small amount in a pill can cause bloating, gas, or diarrhea. That’s not a side effect of the active drug - it’s the filler. Gluten, found in some starches used as binders, can trigger reactions in people with celiac disease. About 1 in 7 people have some level of gluten sensitivity. If your medication contains wheat starch, you might not know it unless you check the label. Sulfites, used as preservatives in injectables, can cause breathing problems in people with asthma. Benzyl alcohol, used in some IV solutions, can be dangerous for newborns. The FDA’s Adverse Event Reporting System shows that around 0.5% of all bad reactions to medicines are caused by inactive ingredients - not the active drug. That might sound small, but with billions of prescriptions filled every year, that’s tens of thousands of people affected.What you should do
You don’t need to be a chemist to protect yourself. Here’s what works:- Read the label. On over-the-counter meds, both active and inactive ingredients are listed on the back. For prescriptions, check the package insert or ask your pharmacist.
- Ask your pharmacist. If you have allergies, intolerances, or dietary restrictions, pharmacists are trained to spot problematic excipients. In 2022, nearly a quarter of medication switches in U.S. pharmacies were due to inactive ingredient concerns - not because the drug wasn’t working.
- Look for alternatives. Many drugs come in multiple forms. If one has lactose and you’re intolerant, there’s probably another brand that uses cornstarch instead. Your pharmacist can help you find it.
- Use the FDA’s Inactive Ingredient Database. It’s public, free, and updated quarterly. You can search by ingredient or drug form to see what’s allowed and in what amounts.

The bigger picture
The pharmaceutical industry is starting to change how it thinks about excipients. The global market for inactive ingredients is expected to hit $11.3 billion by 2027. Why? Because companies now know they’re not just fillers - they’re part of the medicine’s performance. For example, a newer version of the cholesterol drug fenofibrate used special surfactants in its formula. That change boosted how much of the drug your body absorbed by 35%. The active ingredient was the same - but the inactive ingredients made it work better. Australia started requiring doctors to prescribe by active ingredient in 2020. That means instead of saying ‘Prescribe Lipitor,’ they say ‘Prescribe atorvastatin.’ This helps patients get the same drug at a lower cost - and also makes it easier to avoid specific excipients if they’re sensitive. In the U.S., active ingredient prescribing has grown 37% since 2017. More doctors and pharmacists are asking: ‘What’s in this pill besides the drug?’What’s next
The future of medicine might not just be about better drugs - but better delivery systems. Scientists are using AI to predict how excipients interact with human proteins before they’re even put into a pill. Some researchers are pushing to retire the term ‘inactive’ altogether. Maybe we’ll start seeing labels like ‘biologically neutral,’ ‘low-impact,’ or ‘potential interaction risk’ instead. For now, the message is simple: Your medicine isn’t just the active ingredient. The rest matters too. And if you’ve ever had an unexplained reaction - bloating, rash, stomach upset - after taking a pill, it might not be the drug. It might be the filler. Don’t ignore it. Check the label. Ask your pharmacist. Your body will thank you.Are inactive ingredients safe?
Yes - at the levels used in medications. The FDA requires all inactive ingredients to be Generally Recognized As Safe (GRAS) or already listed in their Inactive Ingredient Database. But ‘safe for most people’ doesn’t mean safe for everyone. Some people react to lactose, gluten, dyes, or preservatives in pills. These reactions are rare but real, and they’re often missed because they’re not caused by the active drug.
Can inactive ingredients affect how well a drug works?
Absolutely. The wrong filler or coating can stop your body from absorbing the active ingredient properly. For example, a reformulated version of fenofibrate with better surfactants increased absorption by 35%. That’s not a small change - it’s the difference between a drug working and not working.
How do I know if my medication contains lactose or gluten?
Check the ‘Inactive Ingredients’ section on the drug label or package insert. Lactose, wheat starch, and maltodextrin (which can be wheat-derived) are common culprits. If you’re unsure, ask your pharmacist - they have access to databases that list exact ingredients and sources. Many pharmacies can also order lactose-free or gluten-free versions.
Why don’t doctors talk about inactive ingredients?
Most doctors focus on the active ingredient because that’s what treats the condition. But pharmacists are trained to spot excipient issues. If you have allergies, intolerances, or chronic conditions, bring up excipients when you get a new prescription. Many people don’t realize this is something you can - and should - ask about.
Is there a list of safe inactive ingredients?
Yes. The FDA’s Inactive Ingredient Database lists over 1,000 ingredients approved for use in different drug forms - like oral tablets, injections, or creams - along with maximum allowed amounts. It’s publicly available and updated every three months. Pharmacists use it daily to verify safety and compatibility.



Kathy McDaniel
January 28, 2026 AT 00:17April Williams
January 28, 2026 AT 01:02Harry Henderson
January 28, 2026 AT 13:36astrid cook
January 29, 2026 AT 23:23Andrew Clausen
January 30, 2026 AT 12:36Marian Gilan
January 31, 2026 AT 07:48Desaundrea Morton-Pusey
January 31, 2026 AT 08:02Murphy Game
February 1, 2026 AT 14:21John O'Brien
February 3, 2026 AT 04:13Kegan Powell
February 3, 2026 AT 14:14suhail ahmed
February 5, 2026 AT 07:21Candice Hartley
February 6, 2026 AT 19:30Anjula Jyala
February 7, 2026 AT 06:00Kirstin Santiago
February 8, 2026 AT 09:16