Why drug shortages keep happening-and how to stop them
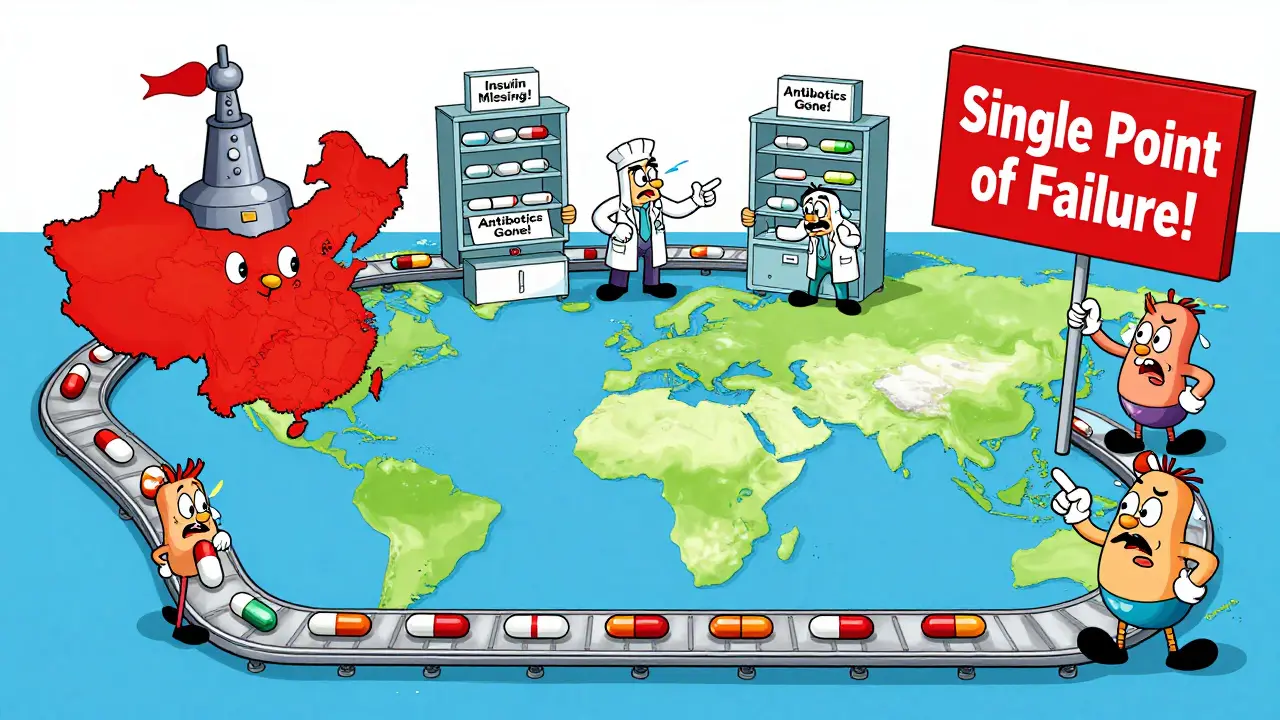
Every year, hospitals in the U.S. face sudden shortages of life-saving drugs. Antibiotics, cancer treatments, insulin, and even basic IV fluids disappear without warning. It’s not bad luck. It’s a broken system. The problem isn’t just about running out of pills-it’s about how those pills are made, where they come from, and who’s responsible when things go wrong. The truth is, most of the active ingredients in your medicine come from just two countries: China and India. Together, they produce nearly 70% of the world’s active pharmaceutical ingredients (APIs). When a factory in Shanghai shuts down for inspection, or monsoon floods hit a plant in Hyderabad, patients in America pay the price. And it’s getting worse.
What resilience actually means in pharma supply chains
Resilience doesn’t mean having more stockpiles. It doesn’t mean blaming foreign suppliers. Real resilience is about building systems that can bend without breaking. According to the U.S. Department of Health and Human Services, a resilient pharmaceutical supply chain can anticipate, respond to, and recover from disruptions while still delivering critical medicines. That’s not a buzzword-it’s a measurable outcome. Companies that built resilience strategies saw 23% higher operational continuity during disruptions. That’s not just good business. It’s a matter of life and death.
Take sterile injectables. In 2023, the U.S. produced only 12% of its own sterile injectable APIs. That means 88% came from overseas. One factory failure, one regulatory delay, one shipping delay-and hospitals start rationing epinephrine or heparin. Resilience means having backup suppliers, not just one source. It means knowing exactly where every ingredient comes from, down to the 12th tier of suppliers. Most companies still don’t even know who makes the chemicals that go into the chemicals they buy. That’s not supply chain management. That’s gambling.
How much of your medicine is made in the U.S.? Not enough
The U.S. government has spent billions trying to bring drug manufacturing home. The CHIPS and Science Act allocated $1.2 billion for pharmaceutical infrastructure. A new executive order in August 2025 created a Strategic Active Pharmaceutical Ingredients Reserve, aiming to stockpile 90 days’ worth of 150 essential medicines by 2027. Sounds good. But here’s the catch: the U.S. still only produces 28% of its essential medicine APIs domestically. For antibiotics, it’s 17%. For sterile injectables, 12%. You can’t fix a 70% gap by building one new factory.
Trying to make everything in America isn’t realistic-and it’s not even the best solution. The National Academies of Sciences warned that over-relying on domestic production could raise drug costs by 20-30% without actually making the system safer. Why? Because putting all your eggs in one basket-even if that basket is in Ohio-creates a new kind of risk. If that single U.S. plant has a fire, a cyberattack, or a labor strike, you’re back to square one. Resilience isn’t about geography. It’s about diversity.
The three pillars of a resilient supply chain
Leading companies don’t guess. They build using three proven pillars: preparedness, response, and recovery.
- Preparedness means knowing your risks before they happen. Top firms map out every supplier, from the raw material vendor to the packaging partner. They run simulations: What if a port in Los Angeles shuts down? What if India bans API exports? What if a key chemical becomes unobtainable? They don’t wait for crises-they prepare for them.
- Response is about acting fast when disruption hits. Companies with integrated data platforms can identify a shortage in 7 days instead of 45. They have pre-negotiated contracts with backup suppliers. They don’t scramble. They switch.
- Recovery is about getting back to normal faster. That means having the right technology in place-like continuous manufacturing systems that can ramp up production in weeks, not years. Traditional batch production takes 18-24 months to scale. Continuous manufacturing can do it in 6.
And here’s what separates the winners: they invest 5-10% of their supply chain budget into resilience. Most companies spend less than 2%. They treat it like insurance. The best companies get a 1.8x return on that investment within three years-just by avoiding the cost of a single major shortage.
Technology that’s changing the game
Old methods won’t fix this. You can’t build resilience with spreadsheets and phone calls. You need tech.
Continuous manufacturing is one of the biggest breakthroughs. Instead of making drugs in big batches that take weeks, continuous systems produce medicine in a steady flow-like a soda fountain instead of a water cooler. It uses 20-25% less energy, cuts waste by 15-20%, and shrinks factory size by 30-40%. The FDA has approved only 12 such facilities so far-out of over 10,000 traditional batch plants. But that’s changing. New FDA guidance in 2025 cut approval times from 3 years to 18 months. More companies will adopt it.
AI-powered risk forecasting is another game-changer. Systems now predict supply disruptions with 85-90% accuracy up to 90 days in advance. They analyze weather patterns, political unrest, shipping delays, and even social media chatter about raw material shortages. One pilot program used AI to predict a key API shortage in India three months before it happened. The company switched suppliers early. No patient went without.
Blockchain traceability helps fight counterfeit drugs. In some regions, up to 10% of medicines are fake. Blockchain lets every step-from the raw chemical to the pharmacy shelf-be tracked and verified. Early trials showed a 70-75% drop in counterfeit incidents. That’s not just safety. It’s trust.
What works in practice: Real strategies, real results
Companies aren’t waiting for government help. They’re acting.
- Dual-sourcing: Leading firms now source 70-80% of critical APIs from at least two different suppliers in different countries. One in India, one in Europe, one in the U.S. No single point of failure.
- Buffer stock: For essential medicines, top companies keep 60-90 days of inventory on hand. That’s not hoarding. That’s responsibility. In 2024, a hospital system in Minnesota kept a 90-day stock of insulin. When a Canadian supplier halted exports, they didn’t panic. They kept giving patients their doses.
- Regional networks: Instead of relying on Asia for everything, companies are building regional hubs. One major pharma firm now has manufacturing in North America, Eastern Europe, and Southeast Asia. If one region fails, the others compensate.
- Executive ownership: The companies that succeed have CEOs who treat supply chain risk like financial risk. They assign budgets. They measure KPIs. They hold teams accountable. Research shows those with strong executive sponsorship are 3.2 times more likely to succeed.

The cost of doing nothing
Some say building resilience is too expensive. But the cost of not doing it? Much higher.
A single major drug shortage can cost a large pharmaceutical company $14.7 million in lost revenue-plus reputational damage, lawsuits, and worse. In 2023, a shortage of a common chemotherapy drug led to delayed treatments for over 12,000 cancer patients in the U.S. Some died waiting. That’s not a business problem. That’s a moral failure.
The global market for pharmaceutical supply chain resilience is projected to grow from $4.2 billion in 2023 to $9.7 billion by 2027. That’s not just consultants selling software. That’s companies realizing they can’t afford to wait. The companies investing now will be the ones that survive the next crisis. The rest will be left behind.
What you can do-whether you’re a patient, provider, or policymaker
You don’t have to be a CEO to care about this.
- If you’re a patient: Ask your pharmacist: “Is this drug made in the U.S.?” If they don’t know, ask why. Demand transparency. Your health depends on it.
- If you’re a doctor or hospital admin: Push your procurement team to require dual-sourcing for critical drugs. Don’t accept “it’s the only one available.” Push for alternatives. Document shortages. Report them to the FDA’s Drug Shortages Program.
- If you’re a policymaker or investor: Support incentives for continuous manufacturing. Fund workforce training for pharmaceutical technicians. Demand that public health funding includes supply chain resilience as a core metric-not just drug prices.
The old model-lean, global, cheap-is over. The new model is resilient, diversified, and slightly more expensive. But it saves lives. And that’s not a cost. That’s the point.
What causes most drug shortages today?
Most drug shortages are caused by disruptions in the supply of active pharmaceutical ingredients (APIs), which are primarily manufactured in China and India. Factory shutdowns, regulatory delays, natural disasters, and export restrictions are common triggers. A single issue at one API supplier can ripple through dozens of drug products.
Is making drugs in the U.S. the solution to shortages?
Not by itself. While increasing U.S. manufacturing helps, over-reliance on domestic production creates new risks-like single-point failures. The best approach is a balanced strategy: boosting domestic capacity for critical drugs while maintaining diversified global sourcing. The goal isn’t to make everything at home, but to make sure no single country or factory controls the entire supply.
How long does it take to build a new pharmaceutical manufacturing plant?
Traditional batch manufacturing plants take 3-5 years to build and certify. New modular or container-based facilities can be deployed in 12-18 months. Continuous manufacturing plants, while more expensive upfront, can be scaled faster and are easier to relocate if needed.
What’s the difference between batch and continuous manufacturing?
Batch manufacturing produces drugs in large, separate lots-like baking cookies one tray at a time. Continuous manufacturing runs drugs through a constant, automated process-like a conveyor belt. Continuous systems use less space, energy, and raw materials, reduce waste, and can adjust output faster. They’re more efficient but require higher initial investment.
How can patients help prevent drug shortages?
Patients can ask questions. Demand to know where their medication is made. Report shortages to the FDA’s Drug Shortages Program. Avoid stockpiling unless advised by a doctor-panic buying worsens shortages. Support policies that fund supply chain resilience, not just lower drug prices.
Are generic drugs more vulnerable to shortages than brand-name drugs?
Yes. Generic drugs often have lower profit margins, so manufacturers cut corners on inventory and diversification. Many generics rely on a single API supplier with no backup. Brand-name companies usually have more resources to build resilience. That’s why shortages hit generics hardest-like antibiotics, insulin, and blood pressure meds.
What’s next for pharmaceutical supply chains?
By 2030, 65-70% of U.S. pharmaceutical needs will come from regional manufacturing networks-not just Asia or the U.S. alone. Domestic production will rise to 35-40%. AI will predict disruptions before they happen. Blockchain will make counterfeit drugs rare. Continuous manufacturing will become the norm, not the exception.
But none of this happens without action. Not from governments alone. Not from CEOs alone. From everyone who relies on medicine to stay alive.



Jessica Salgado
December 16, 2025 AT 16:17Josh Potter
December 18, 2025 AT 09:32Sam Clark
December 19, 2025 AT 08:08Steven Lavoie
December 20, 2025 AT 00:08Chris Van Horn
December 21, 2025 AT 04:52Brooks Beveridge
December 21, 2025 AT 18:38Evelyn Vélez Mejía
December 22, 2025 AT 00:34Victoria Rogers
December 22, 2025 AT 14:52Jane Wei
December 23, 2025 AT 17:18Anna Giakoumakatou
December 25, 2025 AT 12:41Michael Whitaker
December 25, 2025 AT 21:06