When a patient walks into the pharmacy with a prescription for a brand-name drug, the pharmacist’s first thought isn’t always about cost-it’s about consistency. If the patient has been taking the same pill for years, changing the shape, color, or even the filler inside can cause confusion, side effects, or worse-non-adherence. That’s where authorized generics come in. They’re not just cheaper versions of brand-name drugs. They’re the exact same drug, made by the same company, in the same factory, with the same inactive ingredients. And for many patients, they’re the best substitute available.
What Exactly Is an Authorized Generic?
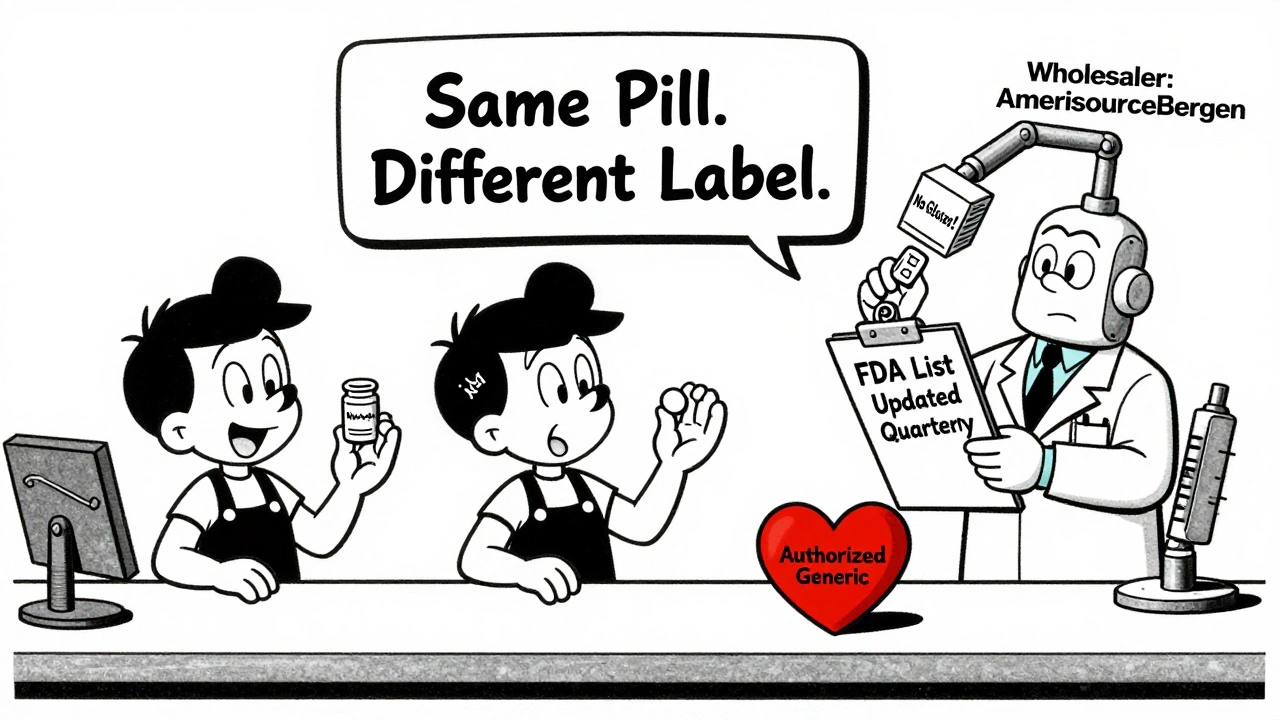
An authorized generic is the brand-name drug sold without the brand name on the label. It’s made by the original manufacturer-or under their direct license-and contains identical active and inactive ingredients to the brand product. Think of it like buying a Coca-Cola bottle with a plain white label instead of the red script. The liquid inside? Exactly the same.
Unlike regular generics, which go through an abbreviated approval process (ANDA) and can have different fillers, coatings, or binders, authorized generics don’t change anything. No reformulation. No testing for bioequivalence-because they don’t need it. They’re the same pill, just packaged differently. The FDA requires manufacturers to list these products quarterly on their website, and as of September 2023, there were 257 authorized generics on the list. That’s about 5% of all brand-name drugs with generic alternatives.
When Should a Pharmacist Recommend an Authorized Generic?
Not every patient needs an authorized generic. But for some, it’s the only safe option. Here are the top three scenarios where pharmacists should actively suggest it:
- Patients with allergies or dietary restrictions-If someone has celiac disease, they can’t tolerate gluten. If they’re vegan, they might avoid gelatin or lactose. Many regular generics use these fillers, but the brand-name version might not. An authorized generic will match the brand’s exact inactive ingredients, making it the only safe generic option.
- Narrow therapeutic index (NTI) drugs-Medications like warfarin, levothyroxine, and phenytoin have very little room for error. A small change in blood levels can lead to dangerous side effects or loss of effectiveness. Studies show that 3-5% of patients on NTI drugs experience issues after switching to a regular generic. Authorized generics eliminate that risk because the formulation hasn’t changed at all.
- Patients who had a bad reaction after switching-If a patient says, “This new pill makes me feel weird,” or “It doesn’t seem to work like before,” don’t assume it’s in their head. A 2021 survey of 1,200 community pharmacists found that 12% of patients reported unexpected side effects or reduced effectiveness after switching to a regular generic. Often, the issue isn’t the active ingredient-it’s the fillers, dyes, or coating. An authorized generic fixes that.
How to Spot an Authorized Generic
It’s not always obvious. Authorized generics often look different from the brand-name version-different color, shape, or markings. But the key is the manufacturer. Check the National Drug Code (NDC) on the bottle. If the labeler code matches the brand-name manufacturer (like Pfizer, Merck, or Novartis), it’s an authorized generic. If it’s a company like Teva or Mylan, it’s a regular generic.
The FDA updates its list of authorized generics every three months. Pharmacists should bookmark it. You can also ask your wholesaler-some distributors, like AmerisourceBergen and Cardinal Health, carry more authorized generics than others. McKesson, for example, tends to stock fewer.
Cost Savings and Insurance Hurdles
Authorized generics usually cost 20-80% less than the brand-name version. That’s more than most patient assistance programs offer. But here’s the catch: insurance plans don’t always treat them like generics.
A 2022 analysis found that 63% of pharmacy benefit managers (PBMs) put authorized generics in the brand-name tier for reimbursement. That means patients could pay more out-of-pocket-even though they’re getting the exact same drug. Pharmacists need to check the patient’s formulary before recommending it. Sometimes, the authorized generic is cheaper. Sometimes, the brand is. Sometimes, the regular generic is. It’s not one-size-fits-all.
Always run the numbers for the patient. Don’t assume. Ask: “What’s your copay for the brand? What’s it for the generic? What’s it for this one?”
Why Patient Counseling Matters
One of the biggest reasons patients stop taking their meds? They think the pill changed because the drug changed. A 2022 study found that 27% of patients discontinued therapy after noticing their pill looked different-unless they were told why.
When you hand someone a new pill, don’t just say, “This is cheaper.” Say: “This is the same medication your doctor prescribed, made by the same company. The only difference is the label. The ingredients inside are exactly the same as your old pill.”
For patients with chronic conditions-diabetes, epilepsy, heart disease-this clarity is critical. It reduces anxiety. It improves adherence. And it prevents avoidable hospital visits.
What Pharmacists Need to Watch Out For
Not every brand has an authorized generic. Only about 5% do. And while most are identical, there are rare cases where a manufacturer tweaks the formulation when launching an authorized version-though this is uncommon and usually disclosed by the FDA.
Also, packaging changes can cause problems. A 2021 study showed that unexpected packaging changes led to 15% of non-adherence cases. If the bottle size changed, the number of pills per pack changed, or the instructions look different, patients get confused. Always explain these changes.
And remember: state laws vary. In 42 states, pharmacists can substitute an authorized generic without prescriber approval-unless the prescription says “Do Not Substitute.” But in 18 states, you must notify the prescriber for any generic substitution, including authorized generics. Know your state’s rules.
The Bigger Picture
Authorized generics aren’t just a cost-saving trick. They’re a precision tool in medication safety. As healthcare shifts toward value-based care, pharmacists who understand these drugs will play a bigger role in preventing adverse events and improving outcomes.
With more patients asking about them-GoodRx reports a 47% jump in searches for “authorized generics” from 2021 to 2022-pharmacists need to be ready. Not just to dispense them, but to explain them, advocate for them, and use them strategically.
It’s not about pushing the cheapest option. It’s about finding the right option-for the patient’s body, their history, their lifestyle, and their peace of mind.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are manufactured by the same company that makes the brand-name drug, using the exact same ingredients-both active and inactive. The only differences are the label, packaging, and sometimes the color or shape of the pill. The medication inside is identical.
Why are authorized generics cheaper than brand-name drugs?
They’re cheaper because they don’t carry the marketing, advertising, or brand-name overhead costs. The manufacturer sells them without the brand name, so they can be priced lower-often 20% to 80% less than the brand. But since they’re made by the same company, the quality and consistency remain unchanged.
Can I substitute an authorized generic without asking the doctor?
In most states, yes-if the prescription doesn’t say “Do Not Substitute.” Forty-two U.S. states allow pharmacists to switch to an authorized generic without contacting the prescriber. But 18 states require notification. Always check your state’s pharmacy board rules before substituting.
Why do some insurance plans charge more for authorized generics?
Many pharmacy benefit managers (PBMs) classify authorized generics under brand-name tiers, not generic tiers, even though the drug is identical. This means patients may pay higher copays than they would for a regular generic. It’s a billing quirk-not a medical one. Always check the patient’s out-of-pocket cost before recommending.
How do I know if a drug has an authorized generic?
Check the FDA’s quarterly list of authorized generics on their website. You can also look at the NDC number on the bottle-if the labeler code matches the brand-name manufacturer (like Pfizer or Merck), it’s an authorized generic. Your wholesaler can also confirm availability.
Are authorized generics safe for patients with food allergies?
Yes-because they use the same inactive ingredients as the brand-name drug. If a patient tolerates the brand because it’s gluten-free, lactose-free, or gelatin-free, the authorized generic will be too. Regular generics often change fillers, which can trigger reactions. Authorized generics eliminate that risk.
Next Steps for Pharmacists
- Bookmark the FDA’s quarterly authorized generics list and check it monthly.
- Train your staff to recognize authorized generics by labeler code, not just appearance.
- Keep a quick-reference chart of common NTI drugs with available authorized generics.
- When a patient reports a problem after switching, ask: “Did you switch to a regular generic or an authorized one?”
- Always counsel patients on why the pill looks different-even if it’s the same drug.
Authorized generics aren’t just another option. For the right patient, they’re the best option. And as a pharmacist, you’re the one who can make that call.





Paul Dixon
December 11, 2025 AT 17:55Man, I wish more pharmacists knew about this. I switched to an authorized generic for my blood pressure med last year and didn’t even notice until my wife pointed out the label was different. No side effects, same results, saved me $40 a month. Why isn’t this common knowledge?
Kristi Pope
December 12, 2025 AT 20:04This is such a quiet win for patient care. Seriously, if you’re a pharmacist reading this-take five minutes to learn the NDC codes. It’s not hard, and you’re not just saving money-you’re saving trust. I’ve seen patients cry because they thought their meds changed. They didn’t. The label did. You’re the bridge. Be the one who explains it like it matters. Because it does.
Katherine Liu-Bevan
December 14, 2025 AT 12:59Authorized generics are the unsung heroes of medication adherence. I’ve counseled elderly patients who refused to take anything that didn’t look exactly like their old pill-even if it was the same active ingredient. Once I showed them the FDA list and explained the manufacturer match, they relaxed. One woman hugged me. That’s the power of clarity. Don’t just dispense. Educate. Always.
john damon
December 16, 2025 AT 03:23Bro, I just realized my thyroid med is an authorized generic now. 🤯 I thought I was getting ripped off but turns out I’m getting the same stuff with no markup. Thanks for the info, I’m telling my whole family. Also, why do PBMs do this? 😡
Courtney Blake
December 17, 2025 AT 12:22Oh great, another ‘pharmacist knows best’ lecture. Let me guess-you also think patients should just trust the system? Newsflash: the same companies that make brand-name drugs also make these ‘authorized’ generics to lock in profits while pretending they’re helping. It’s a marketing ploy dressed up as ethics. And don’t get me started on how PBMs still charge brand prices. You’re not saving anyone-you’re just repackaging greed.
Lisa Stringfellow
December 18, 2025 AT 17:55So… you’re saying if I switch to an authorized generic, I might not die? Wow. That’s… a relief? I guess. But honestly, I’ve been on this med for 12 years and the brand looks like my childhood cereal. Changing it feels like losing a friend. I’m not switching. Sorry.
Taylor Dressler
December 19, 2025 AT 11:15Important clarification: authorized generics are not ‘better’ than regular generics-they’re just identical to the brand. For NTI drugs, that distinction matters. But for many medications, regular generics are perfectly safe. Don’t overstate the case. The goal isn’t to push authorized generics universally-it’s to use them where the risk-benefit ratio justifies it. Precision over promotion.
Monica Evan
December 19, 2025 AT 15:01OMG I just checked my last script and the labeler code is Pfizer-so it’s an authorized generic!! I thought I was getting a knockoff 😅 I’ve been paranoid about this for months. Thanks for explaining how to read the NDC. Now I’m gonna check all my meds. Maybe I’ll finally stop Googling ‘is this pill fake’ at 2am
Jean Claude de La Ronde
December 19, 2025 AT 15:12So let me get this straight: we’ve created a system where the only way to get the exact same drug is to pretend it doesn’t have a brand name? And we call that progress? I’m starting to think capitalism doesn’t want people to be healthy-it just wants them to be confused enough to pay more. 🤷♂️
Mia Kingsley
December 20, 2025 AT 11:14Yeah right. ‘Same ingredients’ my ass. My cousin took an authorized generic for her seizure med and had a seizure. Coincidence? I think not. Also, why are you guys always so sure? Last time you said something was ‘safe’ my dog got sick from a flea pill. Trust me, I know.
Jimmy Kärnfeldt
December 21, 2025 AT 10:18This post gave me chills. I’ve been a pharmacist for 18 years and I’ve seen patients quit meds because they thought the pill changed. This isn’t just about cost-it’s about dignity. When someone’s been on the same drug since their kid was born, that pill is part of their story. You don’t just swap it out. You honor it. And if you can swap it with the exact same thing? That’s not a trick. That’s love.
matthew dendle
December 21, 2025 AT 13:56lol so now pharmacists are supposed to be doctors too? check the ndc code? bookmark the fda list? chill out. most people just wanna pay less and not think about it. you guys are overcomplicating everything. also why is everyone so emotional about pills?
Ariel Nichole
December 23, 2025 AT 00:32Just wanted to say thanks for this. My mom has been on levothyroxine for 20 years and we’ve been terrified to switch. I just checked her bottle-NDC matches the brand. We’re switching to the authorized generic tomorrow. No more $120 copays. And now I know how to explain it to her. You just made her life easier.